Unmet Need
Tolerability Challenges Unnecessary Exposure to
Extended Endocrine Therapy Side Effects Lead
to Noncompliance
The only commercially available test validated to help inform your decision to extend or end endocrine therapy*
Validated by studies of more than 1,500 patients in 3 prospective randomized clinical trial cohorts
LOW Risk/ LOW Likelihood of Benefit
- Consider completion of endocrine treatment at 5 years
- For patients already on extended endocrine treatment, consider stopping endocrine treatment
LOW Risk/ HIGH Likelihood of Benefit
- Consider continuation of endocrine therapy depending on patient tolerability and comorbidities
HIGH Risk/ LOW Likelihood of Benefit
- Consider additional and alternative therapeutic approaches for risk reduction
HIGH Risk/ LOW Likelihood of Benefit
- Consider extended endocrine therapy beyond year 5
- For patients beyond year 5 who are off treatment, consider restarting endocrine treatment
- Emphasize importance of compliance and adherence
on both components of BCI
May support the decision to stop endocrine therapy
on both components of BCI
May support the decision to extend endocrine therapy and encourage tight adherence
BCI helps define your treatment strategy for each patient
- Move from population-based to personalized in the extended endocrine therapy setting
- For patients, not knowing what comes next may be as stressful as initial diagnosis and treatment
- More information may provide your patients with the confidence to take the next step forward
See how BCI benefits patients throughout the treatment journey
BCI Patient ScenariosTour the Test Report
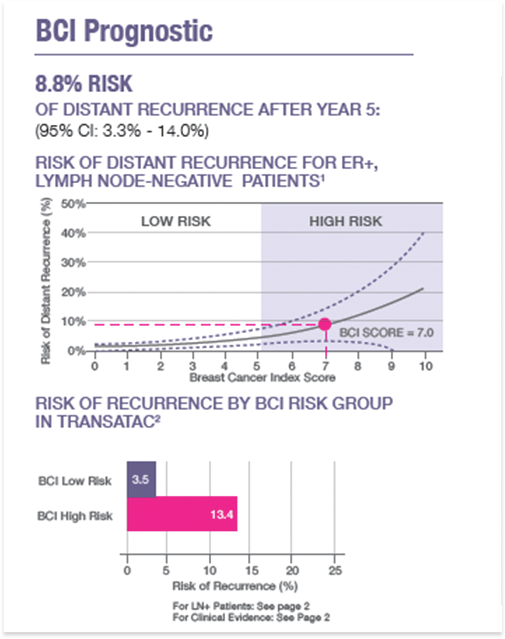
Prognostic Score
- 0 - 10
- Individualized risk of late recurrence*
*If ordered at time of diagnosis, test report also provides overall (years 0-10), early (0-5 year), and late (5-10 year) risk of recurrence
 PROGNOSTIC STUDIES
PROGNOSTIC STUDIES
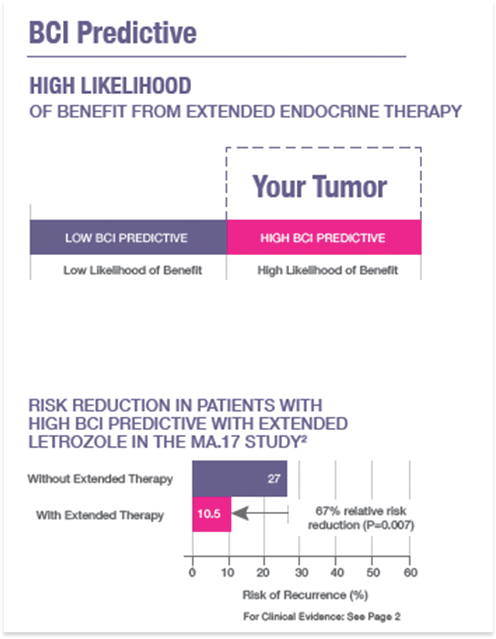
Predictive Score
- Predictive of likelihood of benefit from extended endocrine therapy
- HIGH or LOW (binary result)
 PREDICTIVE STUDIES
PREDICTIVE STUDIES
BCI: clinically actionable information
…BCI might be useful in the setting of late disease recurrence, because it might provide a much needed means of identifying patients who could be spared extended adjuvant endocrine therapy and its well characterised adverse side-effects.
References
- Data on file, bioTheranostics, Inc.
- Sgroi DC, et al. Lancet Oncol. 2013;14:1067 – 76.
Breast Cancer Index Indications for Use and Limitations
BCI provides a quantitative assessment of the likelihood of distant recurrence in patients diagnosed with ER+ node-negative breast cancer, and prediction of likelihood of benefit from extended (>5 year) endocrine therapy in patients who are recurrence-free after an initial 5 years of adjuvant endocrine therapy. Treatment decisions require correlation with all other clinical findings. This test was developed and its performance characteristics determined by bioTheranostics, Inc. lt has not been cleared or approved by the U.S. Food and Drug Administration. This test is used for clinical purposes. lt should not be regarded as investigational or for research. How this information is used to guide patient care is the responsibility of the physician. bioTheranostics is certified under the Clinical Laboratory lmprovement Amendments of 1988 to perform high-complexity clinical laboratory testing.